HURRY
explain the change in the problem using kinetic molecular theory
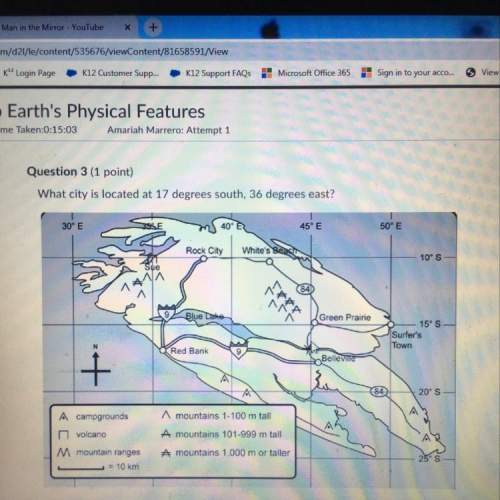
If 22.5 L of nit...

Chemistry, 24.03.2021 03:40 anikabarnhart
HURRY
explain the change in the problem using kinetic molecular theory
If 22.5 L of nitrogen at 0.95 atm are compressed to 1.35 atm at constant temperature, what is the new
volume?
Answer 15.84 L

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, Pizzapegasus1
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 08:30, omoaye
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 23.06.2019 01:30, sheldonwaid4278
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 14.09.2020 06:01

Mathematics, 14.09.2020 06:01

Mathematics, 14.09.2020 06:01

English, 14.09.2020 06:01

Chemistry, 14.09.2020 06:01

Mathematics, 14.09.2020 06:01

Social Studies, 14.09.2020 06:01

Mathematics, 14.09.2020 06:01

Social Studies, 14.09.2020 06:01

Mathematics, 14.09.2020 06:01