
Chemistry, 24.03.2021 01:00 msmojangles
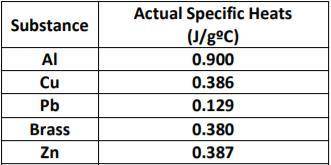
Use the table provided below to help solve the problem.
Sam conducts an experiment to determine an unknown substance. After measuring the unknown substance on a balance, he recorded a mass of 62.5 grams. The initial temperature of the metal was 22oC and that at the end of the experiment the thermometer read 78oC for the metal. His teacher told him that the amount of heat produced was 450.1 joules. Determine the identity of the unknown metal.
You must SHOW WORK and describe how you determined the identity of the unknown metal.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, lwattsstudent
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1


You know the right answer?
Use the table provided below to help solve the problem.
Sam conducts an experiment to determine an...
Questions in other subjects:


History, 13.10.2019 23:50

English, 13.10.2019 23:50




Mathematics, 13.10.2019 23:50

History, 13.10.2019 23:50

Biology, 13.10.2019 23:50

Mathematics, 13.10.2019 23:50



