Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, alexisdiaz365
Your answer should have the same number or significant figures as a he starting measurement. 3201 ml =
Answers: 2

Chemistry, 21.06.2019 19:10, PineaPPle663
Nuclear fusion is the source of energy for stars. besides hydrogen, which other element is most likely also common in stars?
Answers: 1

Chemistry, 22.06.2019 00:00, reeceslife481
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 07:50, mckinleesmomp6qj1e
Which of the following electromagnetic waves can create ions?
Answers: 2
You know the right answer?
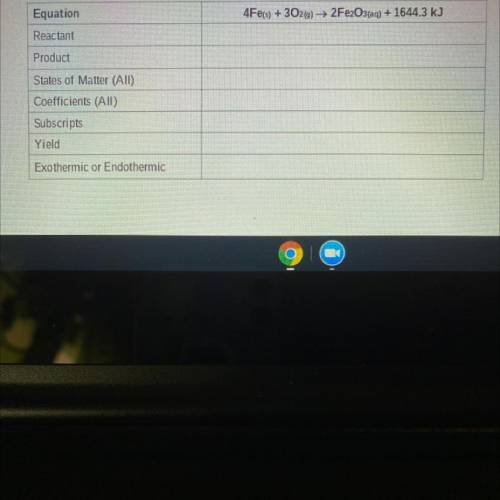
Equation
Reactant
4Fe(s) + 302(g) → 2Fe2O3(aq) + 1644.3 kJ
Product
States of Matt...
4Fe(s) + 302(g) → 2Fe2O3(aq) + 1644.3 kJ
Product
States of Matt...
Questions in other subjects:

Computers and Technology, 08.02.2021 23:50




Computers and Technology, 08.02.2021 23:50

Mathematics, 08.02.2021 23:50



Mathematics, 08.02.2021 23:50