
Chemistry, 23.03.2021 17:20 hannahxmurphs
What is the molality of a solution of hexachlorobenzene dissolved in carbon tetrachloride if the solution has a freezing point of -26.8 °C?
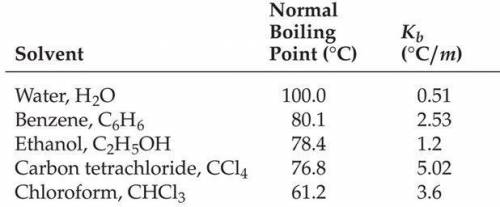

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3


Chemistry, 22.06.2019 22:00, cooljariel11
Give more examples of this type of heat transfer:
Answers: 1
You know the right answer?
What is the molality of a solution of hexachlorobenzene dissolved in carbon tetrachloride if the sol...
Questions in other subjects:



Mathematics, 23.09.2019 09:30

Mathematics, 23.09.2019 09:30




History, 23.09.2019 09:30

Computers and Technology, 23.09.2019 09:30

Health, 23.09.2019 09:30



