
Chemistry, 23.03.2021 02:40 willowcollins3753
1. Above what temperature is it impossible to liquify this substance, no matter what the pressure? 2. At a constant temperature, what would you do to cause this substance to change from the liquid phase to the solid phase?
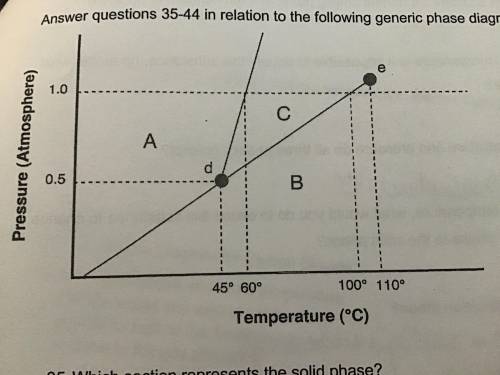

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:10, cefindley14
Which statement is true about the part of the electromagnetic spectrum that human eyes can detect? it contains only the colors of the rainbow and television waves. o it is divided into seven ranges of wavelengths. it contains ultraviolet, visible, and infrared light. it is divided into nine ranges of wavelengths.
Answers: 2

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 07:30, 10040813
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
You know the right answer?
1. Above what temperature is it impossible to liquify this substance, no matter what the pressure?...
Questions in other subjects:

English, 14.10.2020 16:01


French, 14.10.2020 16:01



Health, 14.10.2020 16:01

Biology, 14.10.2020 16:01

Computers and Technology, 14.10.2020 16:01


Physics, 14.10.2020 16:01



