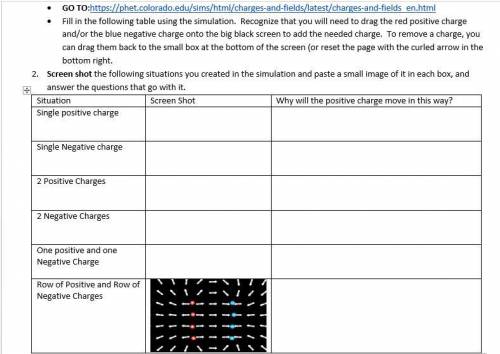
IM not sure how to do this???
the link from the photo is here
https://phet. colorado. edu/si...


Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, mimithurmond03
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1



Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 07.07.2019 01:20


History, 07.07.2019 01:20


Chemistry, 07.07.2019 01:20




Mathematics, 07.07.2019 01:20

Chemistry, 07.07.2019 01:20