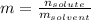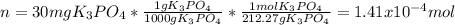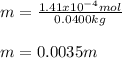Chemistry, 22.03.2021 04:40 trodgers0202
What is the molality of a solution that has 30mg of K3PO4 dissolved in 40mL of water? (The density of water is 1.00 g/mL)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1


Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3
You know the right answer?
What is the molality of a solution that has 30mg of K3PO4 dissolved in 40mL of water? (The density o...
Questions in other subjects:

History, 17.04.2021 05:20

Mathematics, 17.04.2021 05:30





Computers and Technology, 17.04.2021 05:30


English, 17.04.2021 05:30