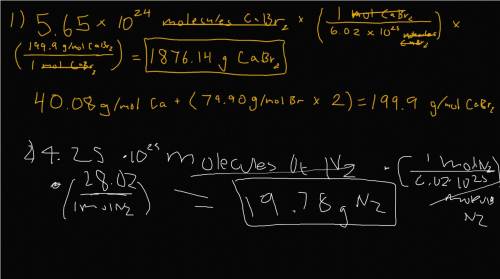Determine the mass of the following.
a) 5.65 x 1024 molecules of CaBr2
b) 4.25 x 1023 molecul...

Chemistry, 22.03.2021 01:50 elijahbravo7107
Determine the mass of the following.
a) 5.65 x 1024 molecules of CaBr2
b) 4.25 x 1023 molecules of N2
c) 9.10 x 1022 molecules of Ba(NO3)2
d) 3.01 x 1022 molecules of Ga2(CO3)3

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, lindseyklewis1p56uvi
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1


Chemistry, 22.06.2019 18:40, bananaslada
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 23:30, ninilizovtskt
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
Questions in other subjects:


Mathematics, 09.11.2020 20:10




Mathematics, 09.11.2020 20:10

History, 09.11.2020 20:10

Mathematics, 09.11.2020 20:10


Mathematics, 09.11.2020 20:10