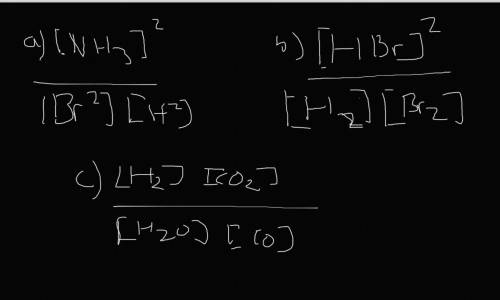Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, citlalli30
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2


Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
Write the expressions for the equilibrium constants of the reactions below. (4 points each)
a. N2...
Questions in other subjects:


Social Studies, 04.02.2021 19:40



History, 04.02.2021 19:40



Mathematics, 04.02.2021 19:40

Mathematics, 04.02.2021 19:40