
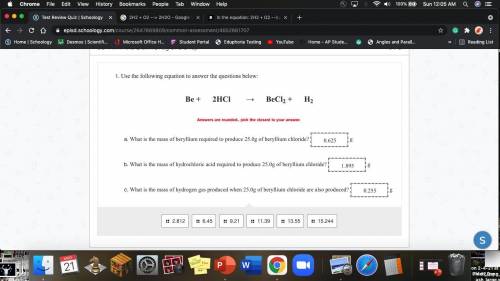
Use the following equation to answer the questions below:
Be + 2HCl → BeCl2 + H2
Answers are rounded.. pick the closest to your answer.
What is the mass of beryllium required to produce 25.0g of beryllium chloride? g
What is the mass of hydrochloric acid required to produce 25.0g of beryllium chloride? g
What is the mass of hydrogen gas produced when 25.0g of beryllium chloride is also produced? g

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, lileljusto2829
Which is the most likely way an automotive engineer would use chemistry
Answers: 1



Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
You know the right answer?
Use the following equation to answer the questions below:
Be + 2HCl → BeCl2 + H2
Answer...
Answer...
Questions in other subjects:

Mathematics, 11.01.2020 22:31

Spanish, 11.01.2020 22:31


Computers and Technology, 11.01.2020 22:31



Computers and Technology, 11.01.2020 22:31

Business, 11.01.2020 22:31


Mathematics, 11.01.2020 22:31



