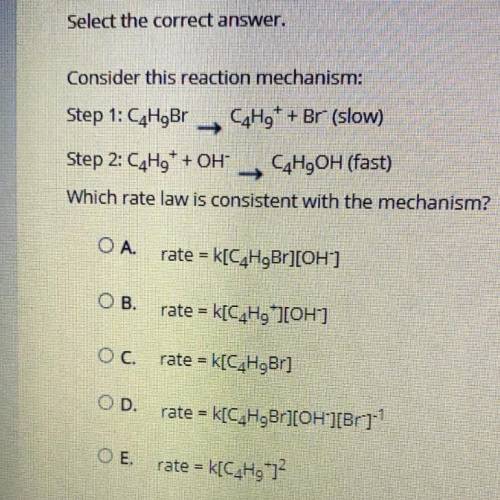
Consider this reaction mechanism:
Step 1: C4H9Br C4H9+ + Br- (slow)
Step 2: C4H9+ + OH- C4H9O...


Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, rscott2649
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 06:00, joelpimentel
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 23.06.2019 01:30, mindofnyny
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1
You know the right answer?
Questions in other subjects:

Business, 18.10.2020 15:01

History, 18.10.2020 15:01




Social Studies, 18.10.2020 15:01


Mathematics, 18.10.2020 15:01


Law, 18.10.2020 15:01