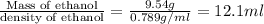Answers: 3


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:30, heavendl13
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1

Chemistry, 23.06.2019 16:00, paigejosie6473
What three relationships are true at equilibrium
Answers: 1

You know the right answer?
The density of ethanol, C2H5OH, is 0.789 g/mL. How many milliliters of ethanol are needed to produce...
Questions in other subjects:

Mathematics, 05.05.2021 21:10




History, 05.05.2021 21:10




Mathematics, 05.05.2021 21:10

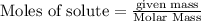
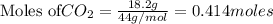
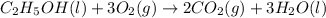
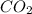 is produced by = 1 mole of
is produced by = 1 mole of 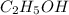
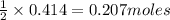 of
of