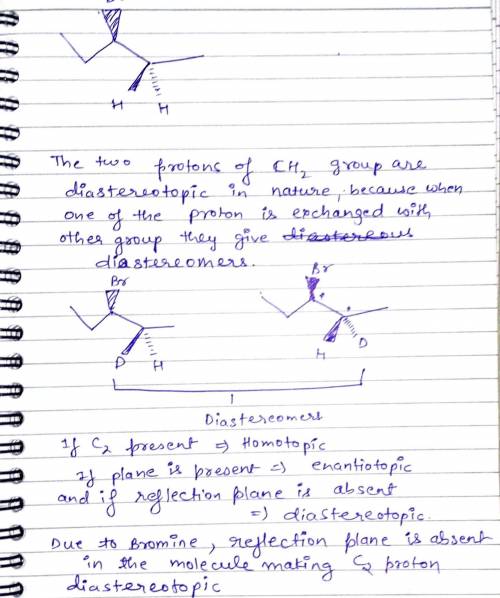
A general rule that the two protons of a CH2 group will be chemically equivalent if there are no chirality centers in the compound. An example of an exception is 3-bromopentane. This compound does not possess a chirality center. Nevertheless, the two highlighted protons are not chemically equivalent. Write down its explaination.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 10:10, alvaradolm6853
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

You know the right answer?
A general rule that the two protons of a CH2 group will be chemically equivalent if there are no chi...
Questions in other subjects:



Mathematics, 10.04.2020 18:35



Mathematics, 10.04.2020 18:35


English, 10.04.2020 18:35


Mathematics, 10.04.2020 18:35