
Acetic acid and water react to form hydronium cation and acetate anion, like this:
HCH3CO2(aq) + H2O(l) → H3O^+ (aq) + CH3CO2^-(aq)
Imagine 226. mmol of CH3CO2- are added to a flask containing a mixture of HCH3CO2, H2O, H3O and CH3CO2- at equilibrium, and then answer the following question.
What is the rate of the reverse reaction before any CH3CO2^- has been added to the flask?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, Brookwiggington8814
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 23.06.2019 00:00, alisonsolis155
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 04:10, nabeelunique
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2
You know the right answer?
Acetic acid and water react to form hydronium cation and acetate anion, like this:
HCH3CO2(aq) + H2...
Questions in other subjects:


Biology, 21.03.2021 06:50


Physics, 21.03.2021 06:50



Mathematics, 21.03.2021 06:50

English, 21.03.2021 06:50

Social Studies, 21.03.2021 06:50

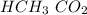 , to which 3 is added. In balance, that rate of the forward reaction was its rate with forwarding reaction, both of which are higher than 0 as the response has achieved balance so that both species get a level greater than 0.
, to which 3 is added. In balance, that rate of the forward reaction was its rate with forwarding reaction, both of which are higher than 0 as the response has achieved balance so that both species get a level greater than 0.


