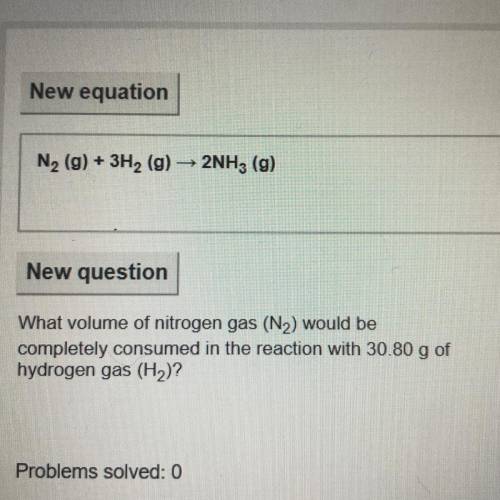
What volume of nitrogen gas (N2) would be
completely consumed in the reaction with 30.80 g of
...


Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, boonkgang6821
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 21:30, sierradanielle9280
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3


Chemistry, 23.06.2019 07:00, asims13
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1
You know the right answer?
Questions in other subjects:

History, 30.06.2019 03:30

Mathematics, 30.06.2019 03:30

Spanish, 30.06.2019 03:30

English, 30.06.2019 03:30

History, 30.06.2019 03:30


Spanish, 30.06.2019 03:30

Mathematics, 30.06.2019 03:30

Social Studies, 30.06.2019 03:30

Chemistry, 30.06.2019 03:30