
Chemistry, 18.03.2021 21:20 vanitycarraway2000
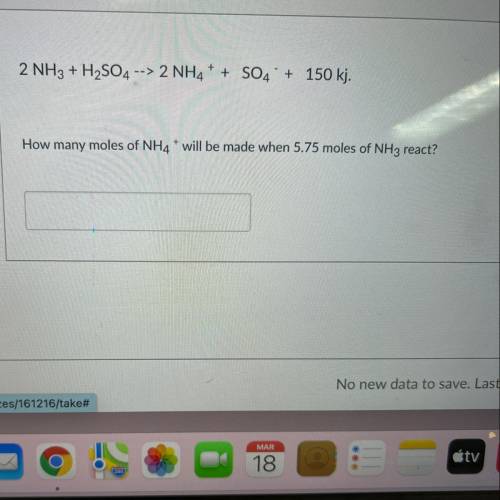
2 NH3 + H2SO4 --> 2NH4+ + SO4 + 150 kj. How many moles of NH4* will be made when 5.75 moles of NH3 react?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, maddynichole2017
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 05:30, jameskarbar9p8c9d2
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1


Chemistry, 22.06.2019 11:40, Wemaybewrong
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1
You know the right answer?
2 NH3 + H2SO4 --> 2NH4+ + SO4 + 150 kj.
How many moles of NH4* will be made when 5.75 moles of N...
Questions in other subjects:

Chemistry, 24.02.2021 20:20


Mathematics, 24.02.2021 20:20




Spanish, 24.02.2021 20:20

Mathematics, 24.02.2021 20:20

Mathematics, 24.02.2021 20:20



