
Chemistry, 18.03.2021 20:50 aroman4511
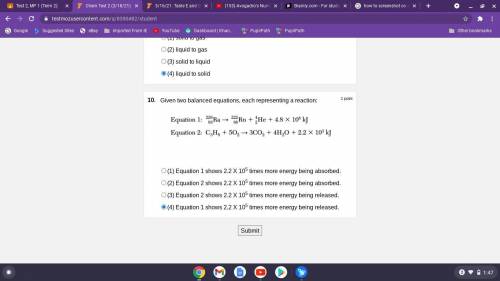
Given two balanced equations, each representing a reaction:
(1) Equation 1 shows 2.2 X 105 times more energy being absorbed. (\
2) Equation 2 shows 2.2 X 105 times more energy being absorbed.
(3) Equation 2 shows 2.2 X 105 times more energy being released.
(4) Equation 1 shows 2.2 X 105 times more energy being released.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, johnnydenali67
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 03:10, josephpezza18
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1


Chemistry, 22.06.2019 23:00, 1315055427
Which subshell is represented by the actinides family?
Answers: 1
You know the right answer?
Given two balanced equations, each representing a reaction:
(1) Equation 1 shows 2.2 X 105 times mo...
Questions in other subjects:

Mathematics, 08.08.2019 08:10


Mathematics, 08.08.2019 08:10


Computers and Technology, 08.08.2019 08:10



Mathematics, 08.08.2019 08:10

Biology, 08.08.2019 08:10



