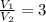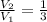Chemistry, 18.03.2021 14:00 nelsoneligwe7
1. The pressure of a gas is 100.0 kPa and its volume is 500.0 ml. If the volume increases to 1,000.0 ml, what is the new pressure of the gas?
2. If a gas at 25.0 °C occupies 3.60 liters at a pressure of 10 kPa, what will be its volume at a pressure of 25 kPa?
3. When the pressure on a gas increases three times, by how much will the volume increase or decrease?
4. Boyle's Law deals what quantities?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:30, camerondillonn
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 23.06.2019 00:30, runninglovexoxo
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

Chemistry, 23.06.2019 01:30, joyelewis58
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
1. The pressure of a gas is 100.0 kPa and its volume is 500.0 ml. If the volume increases to 1,000.0...
Questions in other subjects:

English, 16.02.2020 22:49

Social Studies, 16.02.2020 22:49

Mathematics, 16.02.2020 22:49



History, 16.02.2020 22:51

Mathematics, 16.02.2020 22:51

Mathematics, 16.02.2020 22:51

English, 16.02.2020 22:52

Mathematics, 16.02.2020 22:52

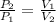 (1)
(1)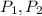 - Initial and final pressure, measured in kPa.
- Initial and final pressure, measured in kPa.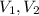 - Initial and final pressure, measured in mililiters.
- Initial and final pressure, measured in mililiters.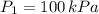 ,
, 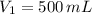 and
and 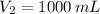 , then the new pressure of the gas is:
, then the new pressure of the gas is: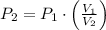
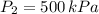
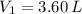 ,
, 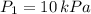 and
and 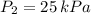 then the new volume of the gas is:
then the new volume of the gas is: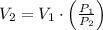
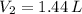
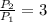 , then the volume ratio is:
, then the volume ratio is: