6HCl(aq) + 2A1)
-
3H2(g) + 2AlCl3(aq)
1. How many moles of hydrogen are produced from 1...

Chemistry, 18.03.2021 09:00 jazzy76783
6HCl(aq) + 2A1)
-
3H2(g) + 2AlCl3(aq)
1. How many moles of hydrogen are produced from 1.20 moles of aluminum?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, TheViperMlg23676
What causes most sediment to wash or fall into a river
Answers: 1

Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
Questions in other subjects:




Mathematics, 03.12.2020 23:50




Mathematics, 03.12.2020 23:50


 will be produced 1.20 moles of aluminium.
will be produced 1.20 moles of aluminium.

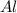 produce = 3 moles of
produce = 3 moles of 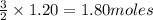 of
of 


