
Chemistry, 18.03.2021 09:10 xelynncaldera
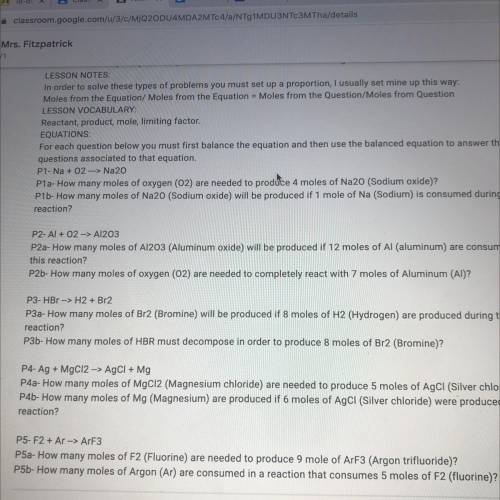
P5-F2 + Ar --> ArF3
P5a- How many moles of F2 (Fluorine) are needed to produce 9 mole of ArF3 (Argon trifluoride)?
P5b- How many moles of Argon (Ar) are consumed in a reaction that consumes 5 moles of F2 (fluorine)?
I need the answers for the p5 part please help

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:00, WinterStrikesBack
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 14:30, Hannahmiller3773
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
P5-F2 + Ar --> ArF3
P5a- How many moles of F2 (Fluorine) are needed to produce 9 mole of ArF3 (A...
Questions in other subjects:








Mathematics, 03.03.2020 01:56

Mathematics, 03.03.2020 01:56

Mathematics, 03.03.2020 01:57



