
Chemistry, 18.03.2021 09:00 destineyburger2
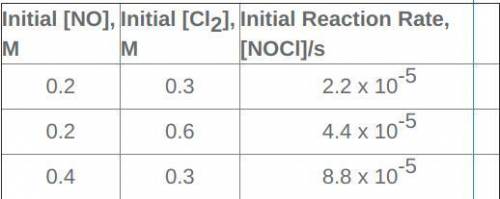
Someone please...help me...seriously I'm gonna breakdown! I can't figure this out for the life of me. I'm gonna cry Given this data, what concentration of each reactant is needed to achieve an initial reaction rate of 1.76 x 10-4 M NOCl/s? Make sure to use the proper units in your answer. Hint: consider how many times bigger this rate is than 2.2 x 10-5 M/s, the first-rate in the rate table.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, matpakootas521
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 21:00, Janznznz4012
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 04:40, twinchristiansp4xhd2
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1

Chemistry, 23.06.2019 05:00, jjoyner
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2
You know the right answer?
Someone please...help me...seriously I'm gonna breakdown! I can't figure this out for the life of me...
Questions in other subjects:

Mathematics, 02.09.2021 23:50


Mathematics, 02.09.2021 23:50

Mathematics, 02.09.2021 23:50


Mathematics, 02.09.2021 23:50

Mathematics, 02.09.2021 23:50



Mathematics, 02.09.2021 23:50



