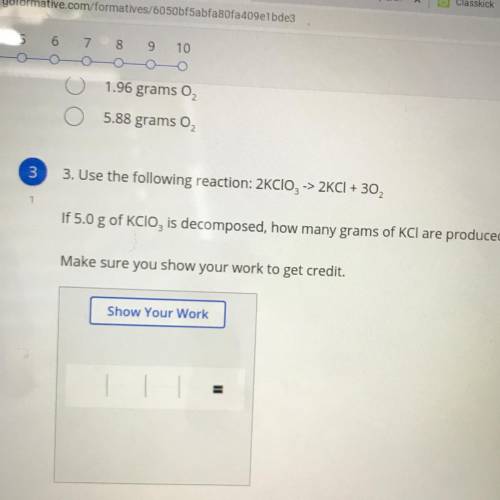
Use the following reaction:
H2SO4+2NaOH—>2H2O+Na2SO4
How many grams of water are produced...

Chemistry, 18.03.2021 03:20 jforeman42
Use the following reaction:
H2SO4+2NaOH—>2H2O+Na2SO4
How many grams of water are produced if 2.0 moles of sodium sulfate are produced?
Make sure you show your work to get credit.
Show Your Work

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 10:30, ciel8809
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 17:20, holmesleauja
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
You know the right answer?
Questions in other subjects:



Mathematics, 11.03.2021 05:10

Mathematics, 11.03.2021 05:10



Mathematics, 11.03.2021 05:10

Mathematics, 11.03.2021 05:10

Mathematics, 11.03.2021 05:10



