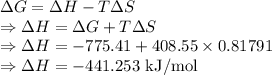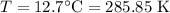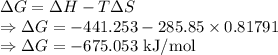Chemistry, 18.03.2021 02:50 Mw3spartan17
For a particular reaction at 135.4 °C, Δ=−775.41 kJ/mol, and Δ=817.91 J/(mol⋅K).
Calculate ΔG for this reaction at 12.7 °C.
Δ=

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 23.06.2019 03:30, rniadsharri16
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1


Chemistry, 23.06.2019 06:10, jamesgotqui6
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a. what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2
You know the right answer?
For a particular reaction at 135.4 °C, Δ=−775.41 kJ/mol, and Δ=817.91 J/(mol⋅K).
Calculate ΔG for t...
Questions in other subjects:


Mathematics, 19.10.2021 14:00


English, 19.10.2021 14:00

Social Studies, 19.10.2021 14:00

English, 19.10.2021 14:00



Mathematics, 19.10.2021 14:00

Mathematics, 19.10.2021 14:00

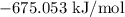
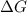 = Gibbs free energy =
= Gibbs free energy = 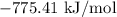
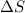 = Change in entropy =
= Change in entropy = 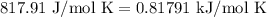
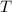 = Temperature =
= Temperature = 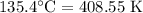
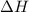 = Change in enthalpy
= Change in enthalpy