
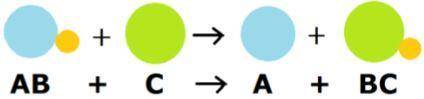
Looking at the image, what does it mean?
I learned: Enthalpy values, Entropy, Calorimetry, Reaction Rates, Equilibrium, and Principle.
My first thought was it is a single replacement reaction, but I feel like there is more to it.
Please no false answers. Thanks much & have a good day!

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, mariamakonteh31
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 22:00, shaylasimonds587
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 10:00, alejandra216
You dissolve 8.65 grams of lead(l) nitrate in water and then you add 2 50 grams of aluminum. this reaction occurs 2ai(s)+ 3pb(no3)2(aq) -3pb(s)+ 2aino3la(aq) the theoretical yield of solid lead?
Answers: 1
You know the right answer?
Looking at the image, what does it mean?
I learned: Enthalpy values, Entropy, Calorimetry, Reaction...
Questions in other subjects:













