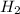Consider the following reaction:
Mg(s) + 2 HCl (aq) → H2(g) + MgCl2(aq)
If 3.65 mol of magnes...

Chemistry, 18.03.2021 01:00 carmencolon119
Consider the following reaction:
Mg(s) + 2 HCl (aq) → H2(g) + MgCl2(aq)
If 3.65 mol of magnesium and 3.65 mol of hydrochloric acid are reacted, how many
moles of hydrogen gas are produced?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, daniel1480
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced. be sure your answer has the correct number of significant digits.
Answers: 2

Chemistry, 22.06.2019 18:30, ashleymer384
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3


Chemistry, 23.06.2019 02:00, matthewsorrow02
What is the mass of 0.750 mole of aluminum oxide, al2o3?
Answers: 1
You know the right answer?
Questions in other subjects:




Mathematics, 01.12.2020 21:50

Mathematics, 01.12.2020 21:50



Mathematics, 01.12.2020 21:50

Social Studies, 01.12.2020 21:50

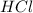 require = 1 mole of
require = 1 mole of 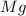
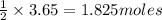 of
of