
Chemistry, 18.03.2021 01:00 angellynn581
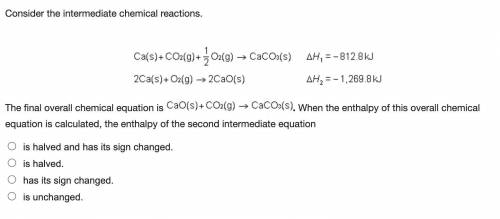
Consider the intermediate chemical reactions.
2 equations. First: upper C a (s) plus upper C upper O subscript 2 (g) plus one half upper O subscript 2 (g) right arrow upper C a upper C upper O subscript 3 (s). Delta H 1 equals negative 812.8 kilojoules. Second: 2 upper C a (s) plus upper O subscript 2 (g) right arrow 2 upper C a upper O (s). Delta H 2 equals negative 1, 269 kilojoules.
The final overall chemical equation is Upper Ca upper O (s) plus upper C upper O subscript 2 (g) right arrow upper C a upper C upper O subscript 3 (s).. When the enthalpy of this overall chemical equation is calculated, the enthalpy of the second intermediate equation
is halved and has its sign changed.
is halved.
has its sign changed.
is unchanged.
pls hurry I will report for wrong answers

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:00, catdog5225
What is formed when amino acids form long chains or polymerize
Answers: 1


Chemistry, 23.06.2019 05:50, kawaunmartinjr10
Aseismic wave is energy released as the result of rock movement along a fault. t or f ?
Answers: 1

Chemistry, 23.06.2019 09:30, carnations
What lessons does the history and study of the periodic table offer to other fields of science, and the pursuit science more generally
Answers: 3
You know the right answer?
Consider the intermediate chemical reactions.
2 equations. First: upper C a (s) plus upper C upper...
Questions in other subjects:

Mathematics, 13.08.2021 06:00

Social Studies, 13.08.2021 06:00

English, 13.08.2021 06:00




Mathematics, 13.08.2021 06:00

English, 13.08.2021 06:00

Mathematics, 13.08.2021 06:00

Social Studies, 13.08.2021 06:00



