
Chemistry, 17.03.2021 23:50 bella122805
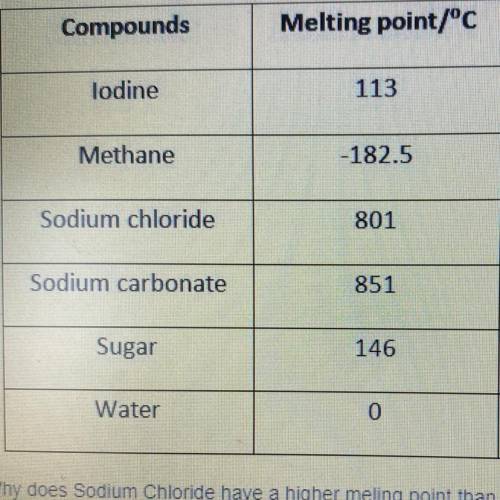
Why does Sodium Chloride have a higher melting point than Sugar?
a. intermolecular forces are weaker
b. intermolecular forces are slightly stronger
c. intermolecular forces are very strong
d. melting point is based on composition and not bonding

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1


Chemistry, 23.06.2019 04:31, saladdressing1425
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1

Chemistry, 23.06.2019 09:00, MrKrinkle77
Individuals within populations exhibit some diversity. as a result of possessing slightly different traits, some individuals are better able to survive and reproduce than others. if these individuals changes in the characteristics of the population may occur over time. the cumulative change in these characteristics is known as
Answers: 3
You know the right answer?
Why does Sodium Chloride have a higher melting point than Sugar?
a. intermolecular forces are weake...
Questions in other subjects:



Mathematics, 13.11.2020 08:40



English, 13.11.2020 08:40

Computers and Technology, 13.11.2020 08:40

Mathematics, 13.11.2020 08:40


Mathematics, 13.11.2020 08:50



