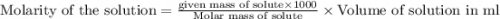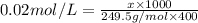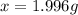Please if you know help
please
the formula of copper sulfate crystals is CuSo4.5H2O. 40...

Chemistry, 13.03.2021 14:00 tiffxnnyyy
Please if you know help
please
the formula of copper sulfate crystals is CuSo4.5H2O. 400ml of 0.02mol/l copper (ii) sulfate solution is needed to be prepered .
a. calculate the molar mass of the solute .
b. calculate the mass of the solute that sould be weighted to prepare this solution .
c. describe in details the steps of preperation of the solution

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, aylengarcia090
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2


Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1
You know the right answer?
Questions in other subjects:


Social Studies, 19.07.2019 17:00

History, 19.07.2019 17:00


Mathematics, 19.07.2019 17:00


Mathematics, 19.07.2019 17:00



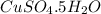 and dissolve in water until the volume is 400 ml
and dissolve in water until the volume is 400 ml