Hydrobromic acid dissolves solid iron according to the
following reaction:
Fe(s) + 2 HBr(aq)...

Chemistry, 13.03.2021 08:10 moranmar2283
Hydrobromic acid dissolves solid iron according to the
following reaction:
Fe(s) + 2 HBr(aq) → FeBr2(aq) + H2(9)
Part A
What mass of HBr (in g) would you need to dissolve a 3.2 - g pure iron
Express your answer using two significant figures.
Part B
What mass of H, would be produced by the complete reaction of the iron

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:40, arlabbe0606
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3


Chemistry, 22.06.2019 14:30, Tooey2331
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3
You know the right answer?
Questions in other subjects:

Spanish, 22.09.2019 18:30

Social Studies, 22.09.2019 18:30


Computers and Technology, 22.09.2019 18:30



Mathematics, 22.09.2019 18:30

Social Studies, 22.09.2019 18:30

Chemistry, 22.09.2019 18:30

Mathematics, 22.09.2019 18:30

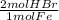 = 0.115 mol HBr
= 0.115 mol HBr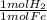 = 0.0573 mol H₂
= 0.0573 mol H₂

