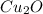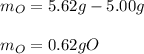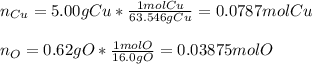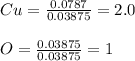Chemistry, 13.03.2021 05:10 robertotugalanp1wlgs
If 5.00 grams of copper metal reacts with oxygen to produce 5.62 grams of 'copper oxide' ,what is the empirical formula of the 'copper oxide' compound?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, claudia122752
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 16:00, hjgjlgkjg
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
If 5.00 grams of copper metal reacts with oxygen to produce 5.62 grams of 'copper oxide' ,what is th...
Questions in other subjects:

Social Studies, 16.10.2020 05:01


History, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01

Advanced Placement (AP), 16.10.2020 05:01


History, 16.10.2020 05:01


Social Studies, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01