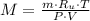Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 18:30, TaraC
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1
You know the right answer?
Determine the molar mass of a 0.314-gram sample of gas having a volume of 1.6 L at 287 K and 0.92 at...
Questions in other subjects:







Medicine, 16.03.2022 06:20


Mathematics, 16.03.2022 06:20

 (1)
(1)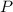 - Pressure, in atmospheres.
- Pressure, in atmospheres. - Volume, in liters.
- Volume, in liters.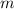 - Mass, in grams.
- Mass, in grams. - Molar mass, in grams per mole.
- Molar mass, in grams per mole. - Temperature, in Kelvin.
- Temperature, in Kelvin. - Ideal gas constant, measured in atmosphere-liters per mol-Kelvin.
- Ideal gas constant, measured in atmosphere-liters per mol-Kelvin.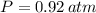 ,
, 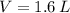 ,
, 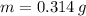 ,
,  and
and 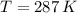 , then the molar mass of the sample is:
, then the molar mass of the sample is: