
Chemistry, 13.03.2021 02:30 eggyhz1980
Please
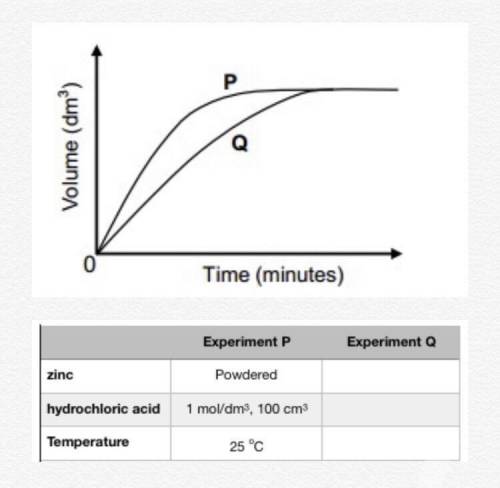
An experiment is carried out to investigate the rate at which hydrogen gas is produced when a given amount of zinc reacts with an excess of a dilute hydrochloric acid solution. Curve P on the graph below shows the volume of hydrogen gas formed when 100 cm3 of a 1 mol/dm3 solution is used. (Reaction rate is indicated by the slope of the graphs.)
Zn + 2 HCl = ZnCl2 + H2
a. Design an experiment that would produce curve Q on the graph below. (Hint: change the experimental parameters so that the rate of gas evolution changes according to the curves). You can also use the table to record your data.
b. What is the implication of the fact that the two curves touch? Explain.
c. Give a reason why the rate of hydrogen production slows down towards the end in both experiments.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, happy121906
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2


You know the right answer?
Please
An experiment is carried out to investigate the rate at which hydrogen gas is produced when...
Questions in other subjects:


Advanced Placement (AP), 06.11.2021 01:30


Biology, 06.11.2021 01:30

English, 06.11.2021 01:30




Physics, 06.11.2021 01:30

History, 06.11.2021 01:30



