Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 20:30, ashley4329
Select all the correct answers. which compounds have the empirical formula ch20? (multiple answers)a. c2h4o2b. c3h603c. ch2o2d. c5h1005e. c6h1206
Answers: 2

Chemistry, 23.06.2019 00:00, sanaiajohnson56
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
You know the right answer?
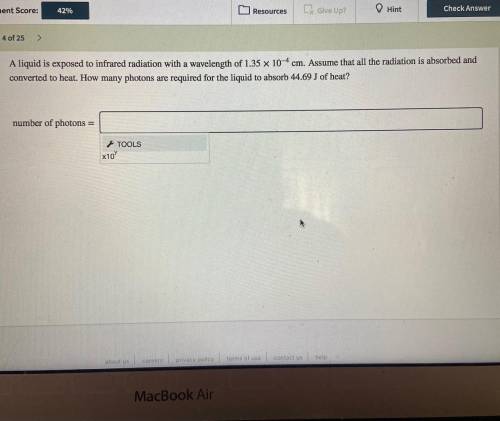
A liquid is exposed to infrared radiation with a wavelength of 1.35×10−4 cm.
Assume that all the ra...
Questions in other subjects:



English, 25.06.2019 18:00

Biology, 25.06.2019 18:00





Chemistry, 25.06.2019 18:00