
Chemistry, 12.03.2021 18:20 savannahvargas512
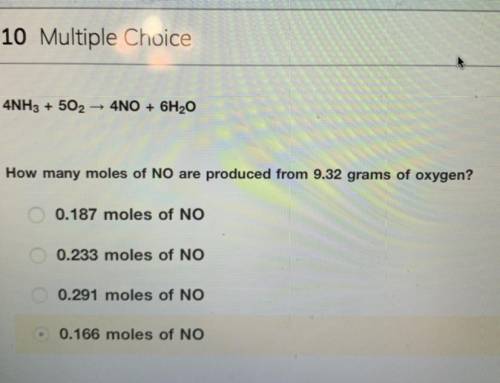
4NH3 + 5O2 > 4NO + 6H2O
How many moles of NO are produced from 9.32 grams of oxygen?
the answer is not 0.166 moles i tried and did not get credit for.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:50, ajaydonlee
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 02:30, sotoamerica0814
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 11:00, hannah5143
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2
You know the right answer?
4NH3 + 5O2 > 4NO + 6H2O
How many moles of NO are produced from 9.32 grams of oxygen?
Questions in other subjects:

Mathematics, 25.11.2021 14:10



Mathematics, 25.11.2021 14:10

Mathematics, 25.11.2021 14:10




History, 25.11.2021 14:10

Business, 25.11.2021 14:10



