Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, mimibear2932
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1


Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3
You know the right answer?
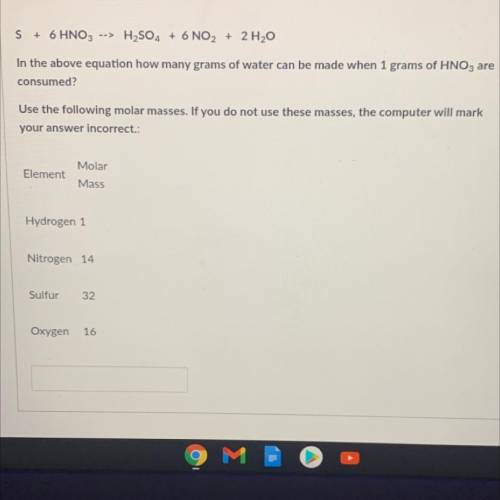
S + 6 HNO3 --> H2SO4 + 6 NO2 + 2 H20
In the above equation how many grams of water can be made w...
Questions in other subjects:

Mathematics, 18.10.2019 17:40





Mathematics, 18.10.2019 17:40