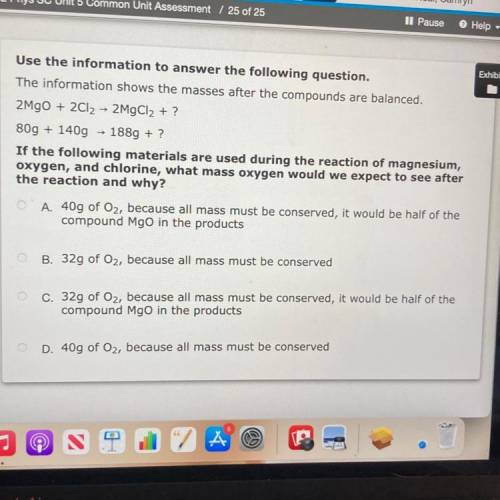
The information shows the masses after the compounds are balanced.
2MgO + 2Cl2 → 2MgCl2 + ?
8...

Chemistry, 11.03.2021 22:30 luthfipadasseri
The information shows the masses after the compounds are balanced.
2MgO + 2Cl2 → 2MgCl2 + ?
80g + 140g + 1889 + ?
If the following materials are used during the reaction of magnesium,
oxygen, and chlorine, what mass oxygen would we expect to see after
the reaction and why?
O A. 40g of Oz, because all mass must be conserved, it would be half of the
compound Mgo in the products
O B. 32g of Oz, because all mass must be conserved
c. 32g of Oz, because all mass must be conserved, it would be half of the
compound Mgo in the products
D. 40g of Oz, because all mass must be conserved

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, ayaanwaseem
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 11:50, tajanaewilliams77
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 20:00, aksambo4707
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
Questions in other subjects:


English, 22.10.2019 04:30











