
Chemistry, 11.03.2021 21:50 itsgiovanna
HELP WILL GIVE BRAINLIEST ASAP
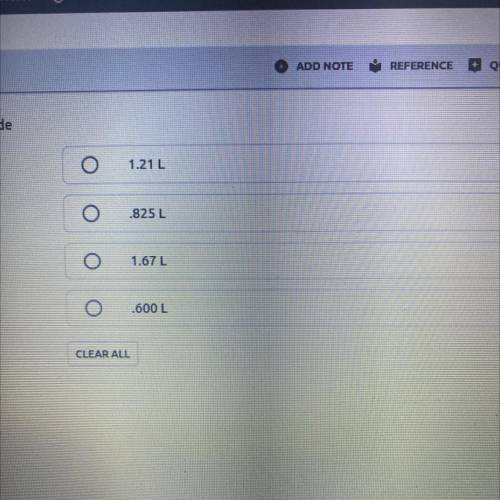
6. Given the combustion reaction CzHg +502 -- 3 C02 + 4 H20, how many liters of carbon dioxide
can be produced from 1.00 liter of Oz at standard temperature and pressure?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, Chynadoll94
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 23.06.2019 05:20, cjking2320
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2
You know the right answer?
HELP WILL GIVE BRAINLIEST ASAP
6. Given the combustion reaction CzHg +502 -- 3 C02 + 4 H20, how man...
Questions in other subjects:

English, 22.01.2021 21:40


History, 22.01.2021 21:40

Health, 22.01.2021 21:40




Mathematics, 22.01.2021 21:40

English, 22.01.2021 21:40

Spanish, 22.01.2021 21:40



