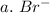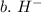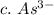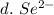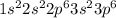a. fe

Chemistry, 02.02.2020 21:47 jazzycintron14
1.* write electron configurations for the 2 + 2 plus cations of these elements.
a. fe
b. co
c. ni
2.write electron configurations for the 3 + 3 plus cations of these elements.
a. chromium
b. manganese
c. iron
3. write the symbol for the ion formed when each element gains electrons and attains a noble-gas electron configuration.
a. br
b. h
c. as
d. se
4.* write electron configurations for the following atoms and ions, and comment on the result.
ar
cl − cap cl to the minus
s 2 − cap s super 2 minus end super
p 3 −

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, MrSavannahCat
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1


Chemistry, 22.06.2019 15:10, kolbehoneyman
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion. what is the frequency f of oscillation?
Answers: 2

You know the right answer?
1.* write electron configurations for the 2 + 2 plus cations of these elements.
a. fe
a. fe
Questions in other subjects:


Chemistry, 11.05.2021 23:40

Mathematics, 11.05.2021 23:40

French, 11.05.2021 23:40





Mathematics, 11.05.2021 23:40

![a.\ Fe^{2+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s 3d^{5} \ or \ [Ar]4s 3d^{5}](/tpl/images/0494/0749/a44c8.png)
![b.\ Co^{2+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s^{2} 3d^{5} \ or \ [Ar]4s^{2} 3d^{5}](/tpl/images/0494/0749/d425b.png)
![c.\ Ni^{2+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s^{2} 3d^{6} \ or \ [Ar]4s^{2} 3d^{6}](/tpl/images/0494/0749/26975.png)
![a.\ Cr^{3+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s^{2} 3d \ or \ [Ar]4s^{2} 3d](/tpl/images/0494/0749/4de3e.png)
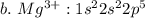
![c.\ Fe^{3+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s^{2} 3d^{3} \ or \ [Ar]4s^{2} 3d^{3}](/tpl/images/0494/0749/51e46.png)