
Chemistry, 11.03.2021 20:40 breeannaalanizj2000
Can someone help me please
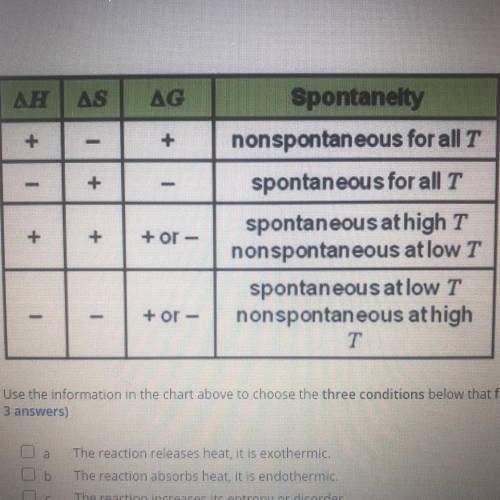
using the information in the chart above to choose the three conditions below that favor spontaneous reaction at all temperaturs ( Choose 3 answers)
A. The reaction release heat it is exothermic
B. The reaction absorbs heat it is endothermic
C. The reaction increases its entropy or disorder
D. The reaction decreases its entropy or disorder
E. The value of G is negative
F. The value of G is positive

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, tot92
Imagine that you’re getting ready to move to a new city. when people move, they are influenced by push factors and pull factors, and you have many reasons for your move. which of the following factors is an example of a pull factor? a. wanting to move because you’ve found a great new school somewhere new b. needing to move because there are not enough resources in your old hometown c. being forced to move because your old home is gone d. having to move because there are no jobs in your current hometown
Answers: 1

Chemistry, 22.06.2019 07:30, deidaraXneji
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3


Chemistry, 22.06.2019 23:30, adamgala3885
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
Can someone help me please
using the information in the chart above to choose the three conditions...
Questions in other subjects:


Mathematics, 16.03.2020 00:28



English, 16.03.2020 00:29

Mathematics, 16.03.2020 00:29

Mathematics, 16.03.2020 00:29

Chemistry, 16.03.2020 00:29

Mathematics, 16.03.2020 00:29



