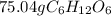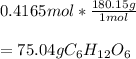Chemistry, 11.03.2021 08:00 skylarladson2549
1. If you have 1.505 X 10^24 atoms of oxygen in glucose (C1,0), how many grams of glucose do you have ?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:20, mgavyn1052
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 14:30, Kiaraboyd9366
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 19:30, toriabrocks
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 21:30, sarah192002
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
You know the right answer?
1. If you have 1.505 X 10^24 atoms of oxygen in glucose (C1,0), how many grams
of glucose do you ha...
Questions in other subjects:


Mathematics, 24.06.2020 19:01

Mathematics, 24.06.2020 19:01



Mathematics, 24.06.2020 19:01

Mathematics, 24.06.2020 19:01