
Chemistry, 11.03.2021 05:50 ilovemusicandreading
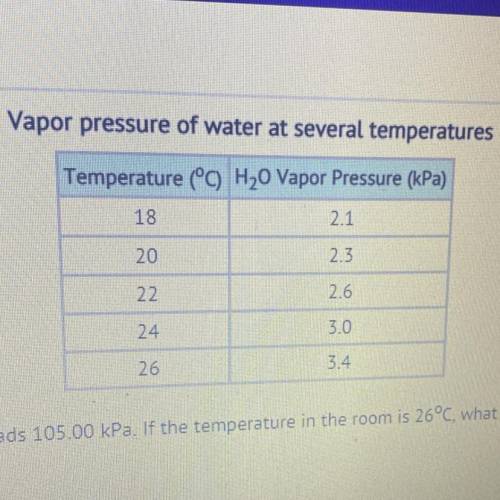
The barometer at an indoor pool reads 105.00 kPa. If the temperature in the room is 26°C, what is the partial pressure of the dry
air??
A
30.88 kPa
B)
101.60 kPa
108.40 kPa
D)
357.00 kPa

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, 767sebmont
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 21.06.2019 22:30, kiki197701
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1

You know the right answer?
The barometer at an indoor pool reads 105.00 kPa. If the temperature in the room is 26°C, what is th...
Questions in other subjects:

English, 09.07.2019 19:10


Mathematics, 09.07.2019 19:10

Mathematics, 09.07.2019 19:10

Health, 09.07.2019 19:10

English, 09.07.2019 19:10

Mathematics, 09.07.2019 19:10





