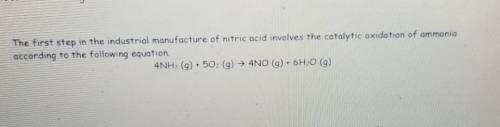Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, letsbestupidcx2314
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 22:20, icantspeakengles
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

You know the right answer?
How Many moles of oxygen(02) are needed to produce 4.6 g of nitrogen monoxide (NO)?
3.36 mol
...
...
Questions in other subjects:

Business, 03.12.2021 07:00


Mathematics, 03.12.2021 07:00

English, 03.12.2021 07:00





English, 03.12.2021 07:00

Mathematics, 03.12.2021 07:00