
Chemistry, 10.03.2021 18:40 esdancer3494
HELP ME PLSS
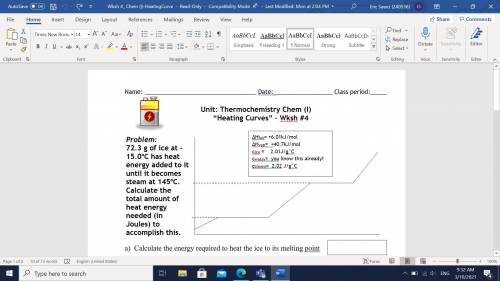
72.3 g of ice at -15.0oC has heat energy added to it until it becomes steam at 145oC. Calculate the total amount of heat energy needed (in Joules) to accomplish this.
A) Calculate the energy required to heat the ice to its melting point?
B) Calculate the energy required to melt the ice?
C) Calculate the energy required to heat the water to its boiling point?
D) Calculate the energy required to vaporize the water?
E) Calculate the energy required to heat the steam to 145oC?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 15:00, kamkam5791
Is powdered sports drink ionic or covalent ? 10pts !
Answers: 1
You know the right answer?
HELP ME PLSS
72.3 g of ice at -15.0oC has heat energy added to it until it becomes steam at 145oC....
Questions in other subjects:

Mathematics, 30.07.2019 02:00

History, 30.07.2019 02:00


History, 30.07.2019 02:00

Biology, 30.07.2019 02:00



History, 30.07.2019 02:00


History, 30.07.2019 02:00



