
Strontium sulfate becomes less soluble in an aqueous solution when sodium sulfator is added because
O the addition of sulfate ions shifts equilibrium to the left.
O the addition of sodium ions shifts equilibrium to the left.
O the addition of sulfate ions shifts equilibrium to the right. Chd
O the addition of sodium ions shifts equilibrium to the right.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:40, ellemarshall13
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2


Chemistry, 22.06.2019 10:10, jojomgarcia01
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 23.06.2019 00:50, trinityine
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
You know the right answer?
Strontium sulfate becomes less soluble in an aqueous solution when sodium sulfator is added because...
Questions in other subjects:






Business, 18.10.2019 04:00


English, 18.10.2019 04:00


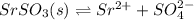
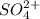 is actually added in to the solution, which causes the equilibrium to shift leftwards according to the Le Ch athelier's principle. Thus, the answer in this case would be:
is actually added in to the solution, which causes the equilibrium to shift leftwards according to the Le Ch athelier's principle. Thus, the answer in this case would be:

