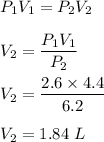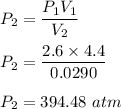Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, gorbyalexis
One of the reactions in a blast furnace used to reduce iron is shown above. how many grams of fe2o3 are required to produce 15.5 g of fe if the reaction occurs in the presence of excess co? a.11.1 g b.22.1 g c.30.0 g d.44.2 g
Answers: 2

Chemistry, 22.06.2019 01:30, jusicca1109
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


Chemistry, 22.06.2019 08:30, itzhari101
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1
You know the right answer?
A sample of air occupies 4.40 L when the pressure is 2.60 atm.
(a) What volume does the sample oc...
Questions in other subjects:


Chemistry, 26.10.2020 17:50

English, 26.10.2020 17:50



Biology, 26.10.2020 17:50



Biology, 26.10.2020 18:00