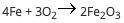Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, haileywebb8
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 15:50, Edwardwall
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 21:20, skyemichellec
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
A student reacts 13 moles of iron with 21 moles of oxygen according to the following equation: (pict...
Questions in other subjects:

English, 30.08.2019 00:10



History, 30.08.2019 00:10


Mathematics, 30.08.2019 00:10

Mathematics, 30.08.2019 00:10

Mathematics, 30.08.2019 00:10