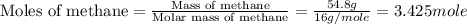Chemistry, 13.10.2019 05:01 averyeverdeen01
The molar heat of vaporization for methane, ch4, is 8.53 kj/mol. how much energy is absorbed when 54.8 g of methane vaporizes at its boiling point?
6.42 kj
29.1 kj
137 kj
467 kj

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, avisconti571
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 18:00, kyllow5644
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 19:50, VoidedAngel
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
The molar heat of vaporization for methane, ch4, is 8.53 kj/mol. how much energy is absorbed when 54...
Questions in other subjects:

English, 01.03.2021 20:50

History, 01.03.2021 20:50

Mathematics, 01.03.2021 20:50

Business, 01.03.2021 20:50

Mathematics, 01.03.2021 20:50

Mathematics, 01.03.2021 20:50

Mathematics, 01.03.2021 20:50

Mathematics, 01.03.2021 20:50

Mathematics, 01.03.2021 20:50