
Chemistry, 09.03.2021 23:50 rockstargirl9245
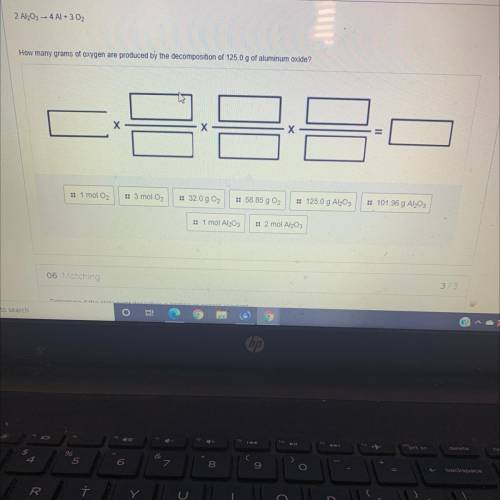
2 Al2O3 4 Al + 3 O2
How many grams of oxygen are produced by the decomposition of 125.0 g of aluminum oxide?
X
Х
Х
:: 1 mol O2
:: 3 mol O2
:: 32.0 g 02
:: 58.85 g 02
:: 125.0 g Al2O3
:: 101.96 g Al203
:: 1 mol Al2O3
:: 2 mol Al2O3

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, JuniperGalaxy
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 14:00, BrandyLeach01
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

You know the right answer?
2 Al2O3 4 Al + 3 O2
How many grams of oxygen are produced by the decomposition of 125.0 g of alumin...
Questions in other subjects:




Mathematics, 14.04.2020 19:02

Social Studies, 14.04.2020 19:02



Computers and Technology, 14.04.2020 19:02





