
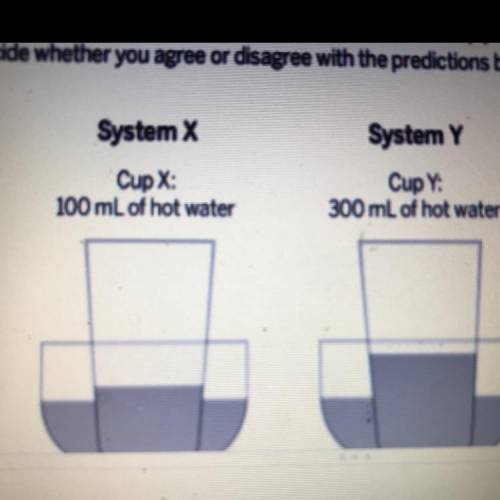
Write whether you agree or disagree with each statement below.
1. The water in Cup X will end up at the same temperature as the water in Container X.
2. The water in Cup Y will end up at the same temperature as the water in Container Y.
3. All of the water in both containers and cups will end at the same temperature.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 17:40, adantrujillo1234
Areaction in which products can react to re-form reactants is
Answers: 1


Chemistry, 23.06.2019 00:00, alisonsolis155
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3
You know the right answer?
Write whether you agree or disagree with each statement below.
1. The water in Cup X will end up at...
Questions in other subjects:

Mathematics, 14.02.2020 01:12

Chemistry, 14.02.2020 01:13



Business, 14.02.2020 01:13

Mathematics, 14.02.2020 01:13


Mathematics, 14.02.2020 01:13




