
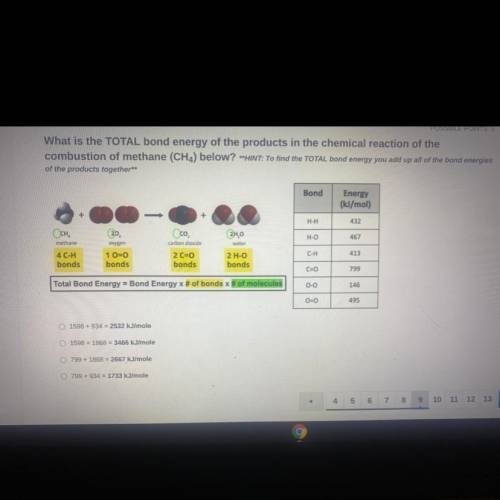
What is the TOTAL bond energy of the products in the chemical reaction of the
combustion of methane (CH4) below? **HINT: To find the TOTAL bond energy you add up all of the bond energies
of the products together**
Bond
Energy
(kJ/mol)
H-H
432
Осн.
H-O
467
methane
Oco
carbon dioxide
2 C=0
bonds
2H,0
water
2 H-O
bonds
oxygen
1 0=0
bonds
413
C-H
4 C-H
bonds
C=0
799
Total Bond Energy = Bond Energy x # of bonds of molecules
0-0
146
00
495
1598 +934 = 2532 kJ/mole
1598 +1868 = 3466 kJ/mole
799 + 1868 = 2667
kJ/mole
799 + 934 = 1733 kJ/mole

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:40, natannale
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1



Chemistry, 23.06.2019 00:50, lakhanir2013
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
You know the right answer?
What is the TOTAL bond energy of the products in the chemical reaction of the
combustion of methane...
Questions in other subjects:

Chemistry, 15.07.2019 07:00

Social Studies, 15.07.2019 07:00

Biology, 15.07.2019 07:00

Chemistry, 15.07.2019 07:00

Geography, 15.07.2019 07:00

Biology, 15.07.2019 07:00

Mathematics, 15.07.2019 07:00

Computers and Technology, 15.07.2019 07:00


English, 15.07.2019 07:00



